
DHEA Derivatives - King of Legal Anabolic Supplements?
If you've been training longer than 10 years, then you are probably familiar with the plethora of prohormones that used to be readily available during the "wild, wild west" era of nutritional supplements. From 1999-2005, it seemed that a new "prohormone" was being released by various companies almost every month, and there were some very strong ones out there.
That party came to a halt in 2005 when the Designer Anabolic Steroid Act of 2004 was implemented. [1]
Then, a few years later people like Patrick Arnold, who famously brought androstendiol and 1-androstendiol (which I'll get to a little later) amongst others, started reading an iconic book about steroids called "Androgens and Anabolic Agents - Chemistry and Pharmacology" by Julius Vida.
This godsend of the chemistry behind anabolic steroids contained literally hundreds of androgens, many that were investigated by pharmaceutical companies but never made it to production, and was responsible for bringing infamous products like Superdrol, DMZ, Epistane, and many many more. The book was very thorough and contained tons of key information about steroids, including the anabolic androgenic ratio (Q) of each compound which indicated how strong each androgen was.
Arguably, this was an even crazier time for prohormones than in the early 2000s. There was no doubt though that this was the era of the "strongest supplements" ever to be on the market, and EVERYONE was getting huge.
Sadly, it came to an end with the introduction of the Designer Anabolic Steroid Control Act of 2014. [2] In the wake of this major blow to the supplement industry and aspiring bodybuilders (like myself) everywhere, was the exclusion of one molecule from this act that would later become the new king of legal anabolic supplements - Dehydroepiandrosterone aka DHEA.

DHEA is one of the most abundant endogenously produced steroids in the body. It is produced in the adrenal glands, gonads, and brain and it considered to be the grandfather of all sex hormones. DHEA is the main intermediate for the biosynthesis of sex hormones like androstenediol, testosterone and estrogen. [3][4]
DHEA has been sold as a nutritional supplement for decades, but it's ability to increase muscle size and testosterone levels in young, weight-trained athletes has been lackluster. [5][6] There are several reasons why this may be the case.
Firstly, DHEA requires not one but two enzymatic reactions to convert to testosterone. The first reaction involves the enzyme 3Beta-hydroxysteroid dehydrogenase (3BHSD) which converts the hydroxyl group on carbon 3 to the necessary ketone group, producing androstendione.
The second step produces testosterone via the reduction of the ketone group on carbon 17 to a hydroxyl group, which is 100% required for binding to the androgen receptor (AR). There is actually a 3rd step that often goes overlooked but this reaction changes the position of the double bond from carbon 5 to carbon 4 via the enzyme -5/-4 isomerase. [7]
To complicate things even further, these enzymes are not present in skeletal muscle tissue, as DHEA/testosterone are not biosynthesized in muscle tissue. DHEA is also the precursor to estradiol and estrone, of which men have lower circulating levels. This may push the reaction further and cause the newly converted testosterone to be aromatized into estrogen as a way for your body to maintain homeostasis and hormonal balance. [8]

So if DHEA doesn't work as a supplement to gain mass, how can it be the king of legal mass builders? Leave it to chemists to figure out the answer. Just how all androgens are derivatives of testosterone and have different anabolic and androgenic effects, there are different naturally-occurring derivatives of DHEA that can dramatically increase its ability to add muscle and increase testosterone levels.
The first one I already mentioned briefly but I will delve into the science behind it now. DHEA has a double bond between carbons 5 and 6, whereas androstenediol and testosterone have a double bond between carbons 4 and 5.
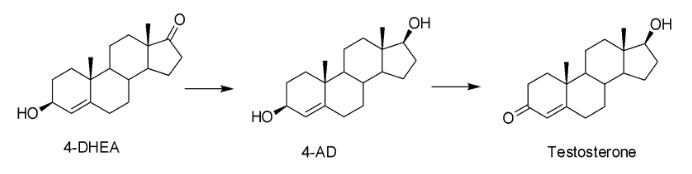
Your body contains the enzyme -5/-4 isomerase which will interconvert (isomerize) the double bond on DHEA before it converts to androstenediol(AD) and testosterone. 4-DHEA is not active on its own and must now go through the two enzymatic steps discussed earlier to convert to testosterone (see reaction below).
Once converted to testosterone, it will now bind to the AR and elicit the same effects as endogenous testosterone would, including increased protein synthesis, decreased protein degradation and increased strength and size. Worth noting, even if some of the 4-DHEA only converts to AD and not testosterone, AD can bind to the AR and is still anabolic on its own.
One of the main benefits of 4-DHEA vs DHEA supplementation is that while 4-DHEA can still convert to estrogen, it cannot directly interact with the estrogen receptor (ER) like DHEA can. DHEA and certain metabolites have been shown to interact with and competitively bind to the ER. [9]
Because 4-DHEA converts to testosterone, it can also undergo aromatization to estrogen and reduction to DHT. If you are taking this supplement, I strongly advise taking an aromatase inhibitor like Arimistane to minimize the conversion to estrogen.
First off, this molecule is now a DHT derivative, meaning there is no double bond on carbon 4-5. The lack of a 4-5 double bond makes the molecule impossible to convert DHT. [10] Just like 4-DHEA, 1-DHEA undergoes the same 2 enzymatic reactions to form the active androgen (see graphic below).

When 1-DHEA undergoes its first enzymatic step it converts into both 1-androstenediol(1-AD) and 1-androstenedione. In theory, neither should directly convert into estrogen, however interestingly 1-androstenedione has been shown to have a higher rate of aromatization and can convert into estrogen. In fact, it a metabolic study of 1-DHEA it was shown that there are 12 metabolites, some of which can also aromatize.
Having said that, 1-DHEA seems to convert to 1-AD 3x more readily than it does to 1-androstenedione, so water retention should be minimal. [11] The final product of these two reactions is 1-testosterone (1-dihydroboldenone) which is significantly stronger than testosterone. According to the Vida book mentioned earlier, 1-testosterone is twice as anabolic as testosterone.
One of the reasons for this may be due to the position of the 1-2 double bond and its ability to protect the 3-keto moiety from being metabolized. In fact, this chemical property is what allows 1-DHEA to have a higher absorption and conversion rate into the parent androgen compared to 4-DHEA.
1-DHEA is also the only one of these newer DHEA derivatives that actually has a clinical study with significant data. In 2013, a study was conducted at the Human Performance Lab at Texas A&M University on 1-DHEA and its anabolic effect on resistance trained athletes.
Seventeen weight trained athletes were given 330mg/d of 1-DHEA or a placebo for 4 weeks and changes in body composition and muscular strength (1RM squat, deadlift and flat bench), as well as lipids and markers for liver and kidney damage were measured. First the good news: In the group receiving 1-DHEA, researchers noticed increased in lean body mass (LBM) of 6.3%, decreased body fat mass by 24.6%, and increased total 1RM by 12.8%.
The placebo group increased LBM by 0.5%, decreased fat mass by 9.5% and increased total 1RM by 5.9%. So as you can see, these were pretty significant changes in muscle mass, fat loss and strength.
Interestingly, these results were very similar to a study conducted on resistance-trained athletes who were given 3.5mg/kg (an average of 300mg/week) of testosterone enanthate (yes, an actual prescription steroid) for 20 weeks! These subjects reported increases in LBM by 6.4%, which is practically the same as the 1-DHEA group. [12] I hope this can really shine light onto the magnitude of this compound that an oral PRECURSOR to an androgen yielded similar results as an injectable steroid.
However, with the good comes the bad and there were some adverse changes in cholesterol levels and markers for liver and kidney health. Subjects who received 1-DHEA on average had a 40% reduction in HDL (good cholesterol), a 30% increase in LDL (bad cholesterol), as well as elevated levels of ALT, AST, and reduction of ALP. Similar findings were reported in the aforementioned testosterone study. [13]
Many are familiar with the anabolic steroid nandrolone (Deca) and its potent ability to add lean body mass with very few side effects. This makes it very attractive to bodybuilders who want to experiment with androgens without engaging in many of the adverse effects these molecules can have.
Well, leave it science to figure out how to take a metabolite of DHEA and turn it into an extremely anabolic compound. 19-Nor-DHEA is a 2 step precursor to nandrolone (nortestosterone), which naturally occurs in the body, albeit in very small quantities. It undergoes the same 2 enzymatic reactions as the other DHEA derivatives mentioned in this article, but the final compound is a very different beast.
The "19-Nor" part of the nomenclature that describes the chemical structure of the molecule. If you compare nandrolone to testosterone, you will see that the methyl group on carbon 19 of the ring structure is missing. This can affect the way nandrolone binds to the AR.
First off, the ring becomes less planar in shape which changes the angle by which the ring structure binds to the AR. It has been hypothesized that because of this angle change, 19-nor compounds are much less androgenic compared to testosterone derivatives. [14]
This is reason behind nandrolone's favorable anabolic ratio, which is 3-6x more anabolic vs testosterone, and its lack of DHT conversion-well in this case its dihydronandrolone- which is much less androgenic than DHT. Nandrolone also converts to estrogen at a much lower rate, about 20% compared to testosterone.


One last unique and mostly anecdotal effect that nandrolone possesses is its ability to help with joint pain. Until recently, I was unsure if this was just an "old gym rat's tale" or if it actually held scientific merit, but I read an article that proposed a mechanism by which nandrolone can help with joint pain.
As mentioned earlier, nandrolone does not readily convert to estrogen so water retention is very low. However, nandrolone can bind to the aldosterone receptor and activate it similarly to aldosterone which is a mineral corticosteroid. Aldosterone controls the flow of sodium and potassium into cells, specifically it brings sodium into your cells and pumps potassium out.
One of the benefits of this is increased fluid retention that can occur in and around your joints. This can help ease joint pain and discomfort and is most likely the reason that users of nandrolone report decreases in joint pain. [15] Nandrolone also has been shown to partially activate the progesterone receptor (PgR).
Progesterone can trigger a response to increase T helper 2 (TH2) cells that can produce anti-inflammatory cytokines and lower the amount of pro-inflammatory cytokines via suppressing T helper 1 cells. Basically your body is increasing natural anti-inflammatory molecules while decreasing production of your body's natural inflammatory response to injury.
This process is known as humoral immunity, and progesterone and other agonists of the PgR (like nandrolone) can stimulate this response. This in turn, may also be another mechanism by which nandrolone may help alleviate joint pain. [16]
Here are some final thoughts that pertain to all 3 of these molecucles:
2) United States Department of Justice; Drug Enforcement Administration; "Rules - 2014-Implementation of The Designer Anabolic Steroid Control Act of 2014"; May 29, 2014
3) Mo Q, Lu SF, Simon NG (April 2006). "Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity". J. Steroid Biochem. Mol. Biol. 99 (1): 50-8.
4) Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK (2006). "The biological actions of dehydroepiandrosterone involves multiple receptors". Drug Metabolism Reviews. 38 (1-2): 89-116.
5) Brown GA, et al. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol. (1999)
6) Ostojic, Sergej M., Julio Calleja, and Morteza Jourkesh. "Effects of short-term dehydroepiandrosterone supplementation on body composition in young athletes." Chinese Journal of Physiology 53.1 (2010): 19-25.
7) Belkien, Lutz, et al. "Pharmacokinetics of 19-nortestosterone esters in normal men." Journal of steroid biochemistry 22.5 (1985): 623-629.
8) Bosy, Thomas Z., Karla A. Moore, and Alphonse Poklis. "The effect of oral dehydroepiandrosterone (DHEA) on the urine testosterone/epitestosterone (T/E) ratio in human male volunteers." Journal of analytical toxicology 22.6 (1998): 455-459.
9) Miller, Kristy K. Michael, et al. "DHEA metabolites activate estrogen receptors alpha and beta." Steroids 78.1 (2013): 15-25.
10) Llewellyn, W; ANABOLICS, 10th Edition; Molecular Nutrition; Amazon Kindle E-Book; August 4, 2011;
11) Parr, Maria K. et al; "Seized Designer Supplement Named -1-Androsterone-: Identification As 3--Hydroxy-5--Androst-1-En-17-One And Its Urinary Elimination;. Steroids; 76.6 (2011): 540-547;
12) Rogerson, Shane, et al. "The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men." Journal of Strength and Conditioning Research 21.2 (2007): 354.
13) Granados, J. et al; "Prohormone Supplement 3 -Hydroxy-5 -Androst-1-En-17-One Enhances Resistance Training Gains But Impairs User Health"; Journal of Applied Physiology; 116.5 (2013): 560-569;
14) Vida, Julius A. "Androgens and anabolic agents: chemistry and pharmacology." (1969).
15) Sekihara, H., Ohsawa, N., & Kosaka, K; "19-Norandrost-4-ene-3, 17-dione amplifies the action of aldosterone only in sodium-loaded conditions: Evidence for a new class of amplifiers of aldosterone"; Biochemical And Biophysical Research Communications; 1980; 93(2), 495-500;
16) Reel, Jerry R., et al. "Competitive progesterone antagonists: receptor binding and biologic activity of testosterone and 19-nortestosterone derivatives." Fertility and sterility 31.5 (1979): 552-561.
That party came to a halt in 2005 when the Designer Anabolic Steroid Act of 2004 was implemented. [1]
Then, a few years later people like Patrick Arnold, who famously brought androstendiol and 1-androstendiol (which I'll get to a little later) amongst others, started reading an iconic book about steroids called "Androgens and Anabolic Agents - Chemistry and Pharmacology" by Julius Vida.
This godsend of the chemistry behind anabolic steroids contained literally hundreds of androgens, many that were investigated by pharmaceutical companies but never made it to production, and was responsible for bringing infamous products like Superdrol, DMZ, Epistane, and many many more. The book was very thorough and contained tons of key information about steroids, including the anabolic androgenic ratio (Q) of each compound which indicated how strong each androgen was.
Arguably, this was an even crazier time for prohormones than in the early 2000s. There was no doubt though that this was the era of the "strongest supplements" ever to be on the market, and EVERYONE was getting huge.
Sadly, it came to an end with the introduction of the Designer Anabolic Steroid Control Act of 2014. [2] In the wake of this major blow to the supplement industry and aspiring bodybuilders (like myself) everywhere, was the exclusion of one molecule from this act that would later become the new king of legal anabolic supplements - Dehydroepiandrosterone aka DHEA.

DHEA is one of the most abundant endogenously produced steroids in the body. It is produced in the adrenal glands, gonads, and brain and it considered to be the grandfather of all sex hormones. DHEA is the main intermediate for the biosynthesis of sex hormones like androstenediol, testosterone and estrogen. [3][4]
DHEA has been sold as a nutritional supplement for decades, but it's ability to increase muscle size and testosterone levels in young, weight-trained athletes has been lackluster. [5][6] There are several reasons why this may be the case.
Firstly, DHEA requires not one but two enzymatic reactions to convert to testosterone. The first reaction involves the enzyme 3Beta-hydroxysteroid dehydrogenase (3BHSD) which converts the hydroxyl group on carbon 3 to the necessary ketone group, producing androstendione.
The second step produces testosterone via the reduction of the ketone group on carbon 17 to a hydroxyl group, which is 100% required for binding to the androgen receptor (AR). There is actually a 3rd step that often goes overlooked but this reaction changes the position of the double bond from carbon 5 to carbon 4 via the enzyme -5/-4 isomerase. [7]
To complicate things even further, these enzymes are not present in skeletal muscle tissue, as DHEA/testosterone are not biosynthesized in muscle tissue. DHEA is also the precursor to estradiol and estrone, of which men have lower circulating levels. This may push the reaction further and cause the newly converted testosterone to be aromatized into estrogen as a way for your body to maintain homeostasis and hormonal balance. [8]

So if DHEA doesn't work as a supplement to gain mass, how can it be the king of legal mass builders? Leave it to chemists to figure out the answer. Just how all androgens are derivatives of testosterone and have different anabolic and androgenic effects, there are different naturally-occurring derivatives of DHEA that can dramatically increase its ability to add muscle and increase testosterone levels.
The first one I already mentioned briefly but I will delve into the science behind it now. DHEA has a double bond between carbons 5 and 6, whereas androstenediol and testosterone have a double bond between carbons 4 and 5.
Your body contains the enzyme -5/-4 isomerase which will interconvert (isomerize) the double bond on DHEA before it converts to androstenediol(AD) and testosterone. 4-DHEA is not active on its own and must now go through the two enzymatic steps discussed earlier to convert to testosterone (see reaction below).
Once converted to testosterone, it will now bind to the AR and elicit the same effects as endogenous testosterone would, including increased protein synthesis, decreased protein degradation and increased strength and size. Worth noting, even if some of the 4-DHEA only converts to AD and not testosterone, AD can bind to the AR and is still anabolic on its own.
One of the main benefits of 4-DHEA vs DHEA supplementation is that while 4-DHEA can still convert to estrogen, it cannot directly interact with the estrogen receptor (ER) like DHEA can. DHEA and certain metabolites have been shown to interact with and competitively bind to the ER. [9]
Because 4-DHEA converts to testosterone, it can also undergo aromatization to estrogen and reduction to DHT. If you are taking this supplement, I strongly advise taking an aromatase inhibitor like Arimistane to minimize the conversion to estrogen.

1-DHEA
Another DHEA derivative that has shown to be much stronger than both 4-DHEA and DHEA is 1-DHEA. The "1" signifies that the double bond is between carbons 1 and 2. This small, but significant change in the molecule drastically changes its properties.First off, this molecule is now a DHT derivative, meaning there is no double bond on carbon 4-5. The lack of a 4-5 double bond makes the molecule impossible to convert DHT. [10] Just like 4-DHEA, 1-DHEA undergoes the same 2 enzymatic reactions to form the active androgen (see graphic below).

When 1-DHEA undergoes its first enzymatic step it converts into both 1-androstenediol(1-AD) and 1-androstenedione. In theory, neither should directly convert into estrogen, however interestingly 1-androstenedione has been shown to have a higher rate of aromatization and can convert into estrogen. In fact, it a metabolic study of 1-DHEA it was shown that there are 12 metabolites, some of which can also aromatize.
Having said that, 1-DHEA seems to convert to 1-AD 3x more readily than it does to 1-androstenedione, so water retention should be minimal. [11] The final product of these two reactions is 1-testosterone (1-dihydroboldenone) which is significantly stronger than testosterone. According to the Vida book mentioned earlier, 1-testosterone is twice as anabolic as testosterone.
One of the reasons for this may be due to the position of the 1-2 double bond and its ability to protect the 3-keto moiety from being metabolized. In fact, this chemical property is what allows 1-DHEA to have a higher absorption and conversion rate into the parent androgen compared to 4-DHEA.
1-DHEA is also the only one of these newer DHEA derivatives that actually has a clinical study with significant data. In 2013, a study was conducted at the Human Performance Lab at Texas A&M University on 1-DHEA and its anabolic effect on resistance trained athletes.
Seventeen weight trained athletes were given 330mg/d of 1-DHEA or a placebo for 4 weeks and changes in body composition and muscular strength (1RM squat, deadlift and flat bench), as well as lipids and markers for liver and kidney damage were measured. First the good news: In the group receiving 1-DHEA, researchers noticed increased in lean body mass (LBM) of 6.3%, decreased body fat mass by 24.6%, and increased total 1RM by 12.8%.
The placebo group increased LBM by 0.5%, decreased fat mass by 9.5% and increased total 1RM by 5.9%. So as you can see, these were pretty significant changes in muscle mass, fat loss and strength.
Interestingly, these results were very similar to a study conducted on resistance-trained athletes who were given 3.5mg/kg (an average of 300mg/week) of testosterone enanthate (yes, an actual prescription steroid) for 20 weeks! These subjects reported increases in LBM by 6.4%, which is practically the same as the 1-DHEA group. [12] I hope this can really shine light onto the magnitude of this compound that an oral PRECURSOR to an androgen yielded similar results as an injectable steroid.
However, with the good comes the bad and there were some adverse changes in cholesterol levels and markers for liver and kidney health. Subjects who received 1-DHEA on average had a 40% reduction in HDL (good cholesterol), a 30% increase in LDL (bad cholesterol), as well as elevated levels of ALT, AST, and reduction of ALP. Similar findings were reported in the aforementioned testosterone study. [13]


Many are familiar with the anabolic steroid nandrolone (Deca) and its potent ability to add lean body mass with very few side effects. This makes it very attractive to bodybuilders who want to experiment with androgens without engaging in many of the adverse effects these molecules can have.
Well, leave it science to figure out how to take a metabolite of DHEA and turn it into an extremely anabolic compound. 19-Nor-DHEA is a 2 step precursor to nandrolone (nortestosterone), which naturally occurs in the body, albeit in very small quantities. It undergoes the same 2 enzymatic reactions as the other DHEA derivatives mentioned in this article, but the final compound is a very different beast.
The "19-Nor" part of the nomenclature that describes the chemical structure of the molecule. If you compare nandrolone to testosterone, you will see that the methyl group on carbon 19 of the ring structure is missing. This can affect the way nandrolone binds to the AR.
First off, the ring becomes less planar in shape which changes the angle by which the ring structure binds to the AR. It has been hypothesized that because of this angle change, 19-nor compounds are much less androgenic compared to testosterone derivatives. [14]
This is reason behind nandrolone's favorable anabolic ratio, which is 3-6x more anabolic vs testosterone, and its lack of DHT conversion-well in this case its dihydronandrolone- which is much less androgenic than DHT. Nandrolone also converts to estrogen at a much lower rate, about 20% compared to testosterone.


One last unique and mostly anecdotal effect that nandrolone possesses is its ability to help with joint pain. Until recently, I was unsure if this was just an "old gym rat's tale" or if it actually held scientific merit, but I read an article that proposed a mechanism by which nandrolone can help with joint pain.
As mentioned earlier, nandrolone does not readily convert to estrogen so water retention is very low. However, nandrolone can bind to the aldosterone receptor and activate it similarly to aldosterone which is a mineral corticosteroid. Aldosterone controls the flow of sodium and potassium into cells, specifically it brings sodium into your cells and pumps potassium out.
One of the benefits of this is increased fluid retention that can occur in and around your joints. This can help ease joint pain and discomfort and is most likely the reason that users of nandrolone report decreases in joint pain. [15] Nandrolone also has been shown to partially activate the progesterone receptor (PgR).
Progesterone can trigger a response to increase T helper 2 (TH2) cells that can produce anti-inflammatory cytokines and lower the amount of pro-inflammatory cytokines via suppressing T helper 1 cells. Basically your body is increasing natural anti-inflammatory molecules while decreasing production of your body's natural inflammatory response to injury.
This process is known as humoral immunity, and progesterone and other agonists of the PgR (like nandrolone) can stimulate this response. This in turn, may also be another mechanism by which nandrolone may help alleviate joint pain. [16]
Here are some final thoughts that pertain to all 3 of these molecucles:
- All of these compounds convert to active steroids. If you are being drug tested, please stay away from them as they may result in failure of your test
- The conversion rate for each one varies, but on average the conversion rate from DHEA derivative to androgen is around 15%. One way to increase this is by buying a supplement that has a liposomal delivery system. This has been shown to significantly increase absorption and conversion(article to come on this)
- Because all of these compounds convert to active steroids, they all have been shown to decrease natural testosterone production, which 19-Nor DHEA also significantly suppressing sperm count. Accordingly, a proper post cycle therapy (PCT) is recommended
- While some more than others, all of these compounds can convert to estrogen. Plan ahead for this and use an aromatase inhibitor or estrogen antagonist
- Like all steroids, these molecules can have adverse effects on your cholesterol levels, and possibly your liver and kidneys. While they are not methylated, 1-DHEA was shown to increase AST and ALT levels so please take a liver and cardio protectant supplement
- These are meant to be taken by males over the age of 18. While females are free to take whatever supplement she chooses, know that these compounds can cause virilization and androgenic side effects
References
1) United States Department of Justice; Drug Enforcement Administration; "Rules - 2005 - Implementation Of The Anabolic Steroid Control Act Of 2004"; December 16, 20052) United States Department of Justice; Drug Enforcement Administration; "Rules - 2014-Implementation of The Designer Anabolic Steroid Control Act of 2014"; May 29, 2014
3) Mo Q, Lu SF, Simon NG (April 2006). "Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity". J. Steroid Biochem. Mol. Biol. 99 (1): 50-8.
4) Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK (2006). "The biological actions of dehydroepiandrosterone involves multiple receptors". Drug Metabolism Reviews. 38 (1-2): 89-116.
5) Brown GA, et al. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol. (1999)
6) Ostojic, Sergej M., Julio Calleja, and Morteza Jourkesh. "Effects of short-term dehydroepiandrosterone supplementation on body composition in young athletes." Chinese Journal of Physiology 53.1 (2010): 19-25.
7) Belkien, Lutz, et al. "Pharmacokinetics of 19-nortestosterone esters in normal men." Journal of steroid biochemistry 22.5 (1985): 623-629.
8) Bosy, Thomas Z., Karla A. Moore, and Alphonse Poklis. "The effect of oral dehydroepiandrosterone (DHEA) on the urine testosterone/epitestosterone (T/E) ratio in human male volunteers." Journal of analytical toxicology 22.6 (1998): 455-459.
9) Miller, Kristy K. Michael, et al. "DHEA metabolites activate estrogen receptors alpha and beta." Steroids 78.1 (2013): 15-25.
10) Llewellyn, W; ANABOLICS, 10th Edition; Molecular Nutrition; Amazon Kindle E-Book; August 4, 2011;
11) Parr, Maria K. et al; "Seized Designer Supplement Named -1-Androsterone-: Identification As 3--Hydroxy-5--Androst-1-En-17-One And Its Urinary Elimination;. Steroids; 76.6 (2011): 540-547;
12) Rogerson, Shane, et al. "The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men." Journal of Strength and Conditioning Research 21.2 (2007): 354.
13) Granados, J. et al; "Prohormone Supplement 3 -Hydroxy-5 -Androst-1-En-17-One Enhances Resistance Training Gains But Impairs User Health"; Journal of Applied Physiology; 116.5 (2013): 560-569;
14) Vida, Julius A. "Androgens and anabolic agents: chemistry and pharmacology." (1969).
15) Sekihara, H., Ohsawa, N., & Kosaka, K; "19-Norandrost-4-ene-3, 17-dione amplifies the action of aldosterone only in sodium-loaded conditions: Evidence for a new class of amplifiers of aldosterone"; Biochemical And Biophysical Research Communications; 1980; 93(2), 495-500;
16) Reel, Jerry R., et al. "Competitive progesterone antagonists: receptor binding and biologic activity of testosterone and 19-nortestosterone derivatives." Fertility and sterility 31.5 (1979): 552-561.

Comments
Leave a comment